What causes intergranular corrosion?
Share
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Please briefly explain why you feel this question should be reported.
Please briefly explain why you feel this answer should be reported.
Please briefly explain why you feel this user should be reported.
When the austenitic stainless steel is heated in the range from 500 to 850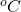 and allowed to cool slowly [i.e welding the austenitic stainless steel and allowing it to cool in the air], the carbon in the grains diffuses to the is grain boundary. Because the diffusivity of carbon is greater than chromium at high temperatures. This is due to the size of carbon is half the size of chromium. Hence carbon diffuses faster. The carbon combines with chromium and forms chromium carbide. Thus, chromium is not available in the grain boundary to form an inert layer called chromium oxide. And also, the chromium layer carbide in the grain boundary acts as a narrow anodic zone and the grains rich in chromium act as a cathodic zone. It results in galvanic coupling and leads to the corrosion between grains i.e., in the grain boundary called inter-granular corrosion.
and allowed to cool slowly [i.e welding the austenitic stainless steel and allowing it to cool in the air], the carbon in the grains diffuses to the is grain boundary. Because the diffusivity of carbon is greater than chromium at high temperatures. This is due to the size of carbon is half the size of chromium. Hence carbon diffuses faster. The carbon combines with chromium and forms chromium carbide. Thus, chromium is not available in the grain boundary to form an inert layer called chromium oxide. And also, the chromium layer carbide in the grain boundary acts as a narrow anodic zone and the grains rich in chromium act as a cathodic zone. It results in galvanic coupling and leads to the corrosion between grains i.e., in the grain boundary called inter-granular corrosion.