Table of Contents
Introduction
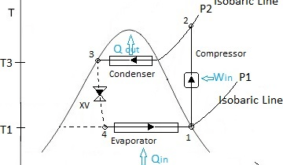
Refrigeration means cooling the space to a temperature lower than ambient temperature. Vapor Compression Refrigeration Cycle (VCRS) is the basic working principle of most of the modern refrigeration systems (i.e. AC, fridges, chillers, air dyer units…etc). The cycle is shown in the figure. A refrigerant is a working fluid in a refrigeration system that absorbs heat from the space which has to be cooled and rejects to the outer atmosphere. The refrigerant circulates in the closed loop. To absorb & throw out the heat to the atmosphere, the refrigerant needs a heat exchanger and to get circulated in the loop it needs a pumping device and to reduce its pressure from P2 to P1 (See the figure) a pressure dropping device or expansion device is needed. Thus, our refrigeration cycle consists of heat exchangers, pumping & pressure dropping device as basic components.
The following figure represents the various thermodynamic states of refrigerant in the ideal vapor compression refrigeration cycle.
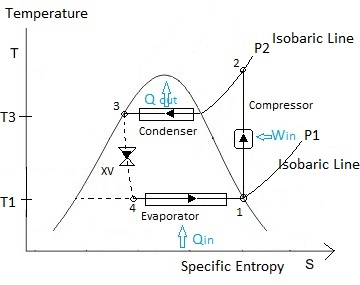
About Figure :
Note: Numbering started from compressor since the cycle starts and ends with compressor as per convention.
Q represents the rate of heat transfer in the above figure.
XV represents the expansion valve.
Path 3-4 in the above figure is represented in dotted line since throttling is an irreversible process.
| Variable | Description |
| P1 | Pressure of refrigerant in the evaporator |
| P2 | Pressure of refrigerant in the condenser |
| T1 | Temperature of refrigerant in/out of the evaporator |
| T3 | Temperature of refrigerant at outlet of the condenser |
| Q in | Rate of heat absorbed from the refrigerated space |
| Q out | Rate of heat rejected to the atmosphere |
| Win | Amount of work input given to the compressor |
Four Basic components of Ideal Vapor Compression Refrigeration Cycle
1. Evaporator
Thermodynamic State or condition of Refrigerant in the evaporator
| At inlet of evaporator (State-4 in fig) | Mixture state (combination of liquid & vapor state) |
| At outlet of evaporator (State-1 in fig): | Saturated Vapor |
An evaporator is a heat exchanger where refrigerant get evaporated by absorbing the heat from the space which has to be cooled and become complete vapor. The temperature of the refrigerant in the evaporator will be very less as compared to the refrigerated space (Of course, we know that heat always flows from high temperature to low temperature). An evaporator can be an indoor unit in case of a split Air conditioner in the houses or it can be a storage space in the case of a refrigerator. In a vapor compression refrigeration cycle the lowest temperature of refrigerant occurs in the evaporator.
2. Compressor
Thermodynamic State or condition of Refrigerant in the compressor
| At inlet of compressor (State-1 in fig) | Low Pressure Saturated Vapor |
| At outlet of compressor (State-2 in fig): | High Pressure Superheated vapor |
The compressor is an external pumping device that compresses the refrigerant from evaporator pressure to condenser pressure. As a result of compression, refrigerant temperature also increases. The refrigerant temperature in the condenser must be higher than the condenser’s cooling media temperature. Refrigerant is compressed to higher pressure because as pressure increases, refrigerant saturation point also increases so that it can be easily cooled to a liquid state in the condenser (state-3 in the figure). The highest temperature of the refrigerant in this cycle occurs at the exit of a compressor.
3. Condenser
Thermodynamic State or condition of Refrigerant in the condenser
| At inlet of condenser (State-2 in fig) | Superheated vapor |
| At outlet of condenser (State-3 in fig): | Saturated Liquid |
A condenser is a heat exchanger where refrigerant rejects heat to the atmosphere and gets condensed to a saturated liquid. Ideally, the condenser converts all the refrigerant vapor into saturated liquid by rejecting the latent heat to the atmosphere due to temperature differences. (Temperature difference between condenser and ambient atmosphere). The condenser can be an outdoor unit in case of a split Air conditioner. Refrigerant pressure in the condenser is constant since it involves phase change from vapor to a liquid state. In a vapor compression refrigeration cycle, refrigerant occurs as a liquid state at the exit of a condenser.
4. Throttling cum Expansion valve
Thermodynamic State or condition of Refrigerant in the expansion valve
| At inlet of expansion valve (State-3 in fig) | Saturated Liquid |
| At outlet of expansion valve (State-4 in fig): | Mixture state (combination of liquid & vapor state) |
Refrigerant has to be brought back from condenser pressure to evaporator pressure so that its temperature will be less than the temperature of the space which has to be cooled. The expansion valve throttles the metered quantity of refrigerant to the evaporator by sensing the heat load in the refrigerated space. Even ideal vapor compression refrigeration cycle involve internal irreversibility in throttling process.
From Darcy – Weisbach equation of head loss, head loss = ( f L V2)/ ( 2 g d)
Where d is the diameter of the pipe. By reducing the pipe diameter, pressure drop increases.
So capillary tube of a very small diameter is used to throttle the refrigerant from condenser pressure to evaporator pressure. Throttling is a constant enthalpy process (H= U + PV). It means that the total energy before and after throttling will be constant.
FAQ
Why it is called vapor compression?
Because the state of refrigerant before entering the compressor is vapor. So obviously the vapor gets compressed in the compressor.
Why refrigerant should be in vapor state in the circuit? Why can’t we maintain the same liquid state throughout the circuit since the thermal conductivity of the liquid is higher than the vapor? Or Why low boiling point fluids are chosen as refrigerants?
Maintaining vapor state in the circuit is not our intention. We aim to maintain the temperature of the refrigerant in the evaporator as low as possible since as per Newton’s law of cooling Q=h A ∆T. (∆T is the temperature difference between the refrigerant and the space that to be cooled). So, if ∆T is more, then faster will be the heat transfer. Next thing, liquid have higher thermal conductivity than vapor. Conductivity tells how well the material can conduct the heat through it. Finally, to achieve the low temperature as well as liquid state of the refrigerant at the evaporator, low boiling point fluids are the optimum choice. Fluids like water cannot be used as refrigerant beyond 0°C due to freezing problem.