Table of Contents
Introduction
Different types of steel and their classification chart is discussed here. Each type of steel is discussed in brief with its unique properties and real-world applications. The image shown above is the golden gate bridge. The beam plates of the golden gate bridge is made of steel.
Steel
Steel is an alloy of iron (metal) and carbon (non-metal) where iron is a primary constituent and carbon is an alloying element. Iron having less than 2 % carbon content by weight is called steel and having more than 2 % of carbon is called cast iron.
Types of Steel
Steel is primarily classified into low and high alloy steel based on the weight percentage of alloying elements added to the steel. Further classification of steel is based on the amount of carbon and other alloying elements present in the steel. The following figure represents the classification of steel.
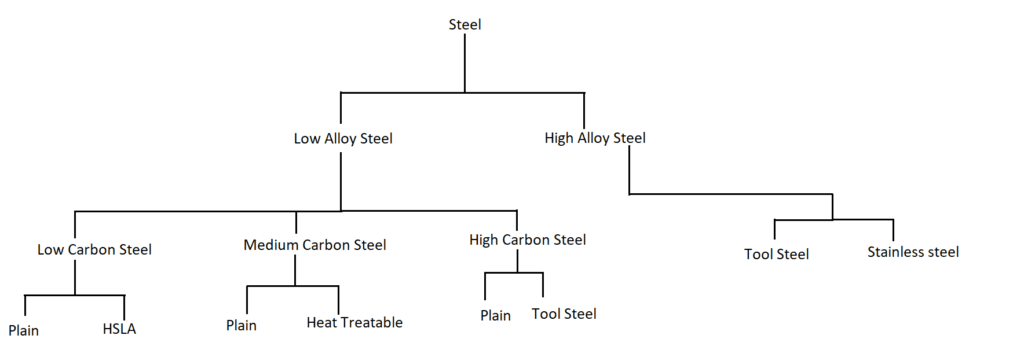
1.Low Alloy Steel
Steel having an alloying element of content less than 8 % by weight is called low alloy steel. Further classification of low alloy steel is based on the amount of carbon content in the steel.
1.1.Low Carbon Steel
- Carbon content in low carbon steel is relatively lower than that of medium and high carbon steel.
- The maximum carbon content in this steel is 0.25 % by weight.
- Mild steel is also known as low-carbon steel.
- Cannot be hardened using classical heat treatment methods like heating and sudden quenching (i.e., the martensitic structure cannot be attained).
Properties of Low Carbon Steel
- Relatively low strength (Ultimate Tensile Strength) as compared to medium and high carbon steel due to less amount of carbon in the steel.
- Highly ductile and tougher.
- Easily weldable and malleable (able to get hammered without fracture).
Applications of Low Carbon Steel
Automobile body parts/ body panels: Body parts of automobiles must be tougher and ductile to get deformed by absorbing the impact load during the crash of a vehicle so that the passenger is safe from high impact energy.
1.1.1.High Strength Low Alloy Steel (HSLA)
HSLA steel has relatively higher strength as compared to its counterpart i.e. mild steel. The reason behind comparing the HSLA steel with mild steel is because both are low carbon steels. Though the carbon content is less, the high strength of HSLA is due to the following strengthening mechanisms.
- Solid solution strengthening
- Precipitation strengthening
- Work hardening
- Grain size refinement.
Anyone or a combination of the above mechanisms can be utilized to get the high strength to low alloy steels. These mechanisms are achieved by using various combinations of alloying elements in steel. Those alloying elements are predominantly niobium, chromium, vanadium, nickel, titanium, copper. Though these alloying elements are added, it is called low alloy steel because they are added in very low amounts. The aim of HSLA steel is not to meet specific chemical composition like other types of steel but intention is to meet specific mechanical properties.
Properties of HSLA steel as compared to mild steel
- High strength to weight ratio
- Good corrosion properties
- Since it is low carbon steel, it has good ductility and formability.
1.2.Medium Carbon Steel
- Carbon content in medium carbon steel is in between low and high carbon steel.
- Carbon content in this steel ranges from 0.25 to 0.6 % by weight.
- Can be hardened using the heat treatment methods but with low hardenability. Thus, only thin sections can be heat treated.
Note: Hardenability is a measure of the depth of martensitic structure from the surface in a ferrous alloy upon quenching.
Properties of Medium Carbon Steel
- Relatively higher strength as compared to low carbon steel.
- Relatively lower ductility as compared to low carbon steel.
- Relatively higher wear resistance property as compared to low carbon steel.
Applications of medium Carbon Steel
Gears: Teeth should be harder to resist wear due to teeth engagement at the same time gear should be tougher to absorb the shock load and strong enough to withstand the load. (i.e., the combination of toughness, hardness & strength).
Railway Tracks: The track surface should be harder to resist the wear due to the passage of wheels and tougher enough to absorb the shock load due to the passage of a fast and bulky train.
1.3.High Carbon Steel
- Carbon percentage is highest among low and medium carbon steel.
- Carbon percentage in this steel ranges from 0.6 to 1.4 % by weight.
Properties of high carbon steel
- Hardest, strongest and least ductile as compared to low and medium carbon steel.
- Cold working cannot be possible since it is hard and strong.
- The higher the carbon content, the higher will be the strength, hardness and wear resistance.
Note: Harder metal will have low ductility and higher brittleness.
Applications of High Carbon Steel
Drill bits, Razor Blades, Axe, and Knives: The cutting edge must be harder to withstand the wear, scratches, and dents while performing a cutting operation.
Note: All plain carbon steel is classified under low alloy steel because theoretically, the maximum carbon that can be added to steel is 6.67 % by weight.
1.3.1.Plain Carbon Steel
Steel that contains only carbon as an alloying element without any other elements added intentionally is called plain carbon steel. But the presence of impurities like silicon, phosphorus…etc in steel is inevitable.
2.High Alloy Steel
Steel having an alloying element content of more than 8 % by weight is called high steel.
2.1.Stainless Steel
A stain is nothing but dirt or a permanent mark which is difficult to remove just like dirt on clothes. Similarly, corrosion is a stain for steel. Stainless steel means corrosion-less steel. The stainless property of the steel is due to the presence of a protective passive layer on the surface of the steel. It is called the passive layer because it is not willing to react with the adjacent media (Atmosphere or any other contact medium).
The passive layer is nothing but a chromium oxide (Cr2O3) present on the surface of stainless steel which is too thin to be visible. The passive layer acts as a shield between the inner surface of metal and external media or ambient atmosphere thus preventing corrosion. To achieve the formation of the chromium oxide layer, the steel should have a minimum of 10.5 to 11 % chromium by weight. So that the chromium on the upper surface of the metal will get oxidized by reacting with oxygen in the atmosphere to form a chromium oxide layer.
Unique Properties of Passive Layer (Cr2O3)
- The chromium oxide layer is inert to most of the chemicals thus, making it a predominant material in the chemical industry and food processing industry so that there is no cross-contamination of food or product due to contact material.
- It has self-healing property. i.e., if there is a loss of passive layer on the surface due to scratches or dents, the chromium present immediately to the bottom of a damaged layer will get reacted to the oxygen to form the chromium oxide layer again.
- It is non-porous. This property makes it a predominant metal in the nuclear industry such that radioactive material spillage on the surface can be cleaned easily and completely without any traces left. Otherwise, the tiny radioactive atoms will settle in the fine pores and start radiating the energy till its lifetime making the whole surface a radioactive source.