Table of Contents
Pitting Corrosion Definition
Pitting corrosion of stainless steel is defined as the localized breakdown of the protective/passive layer (i.e. Chromium oxide layer) on the sensitized metallic surface due to corrosive action which is a result of the reaction between halogen ions & the stainless steel metal that leads to the formation of pits on the surface of the metal. The importance given here is for pitting corrosion in passivating metals (i.e. stainless steel) as compared to non-passivating metals because pitting corrosion is more severe in passivating metals than non-passivating metals.
Pitting Corrosion Pictures

Causes of Pitting Corrosion of Stainless Steel
There are three major causes for pitting corrosion of stainless steel which are as follows.
- Lack of homogeneity in the passive film (or) breaks & metal inclusions in the passive film.
- The presence of halogen ions like Bromide, Chloride, Iodide and fluoride ions in the fluids in contact with the metal surface.
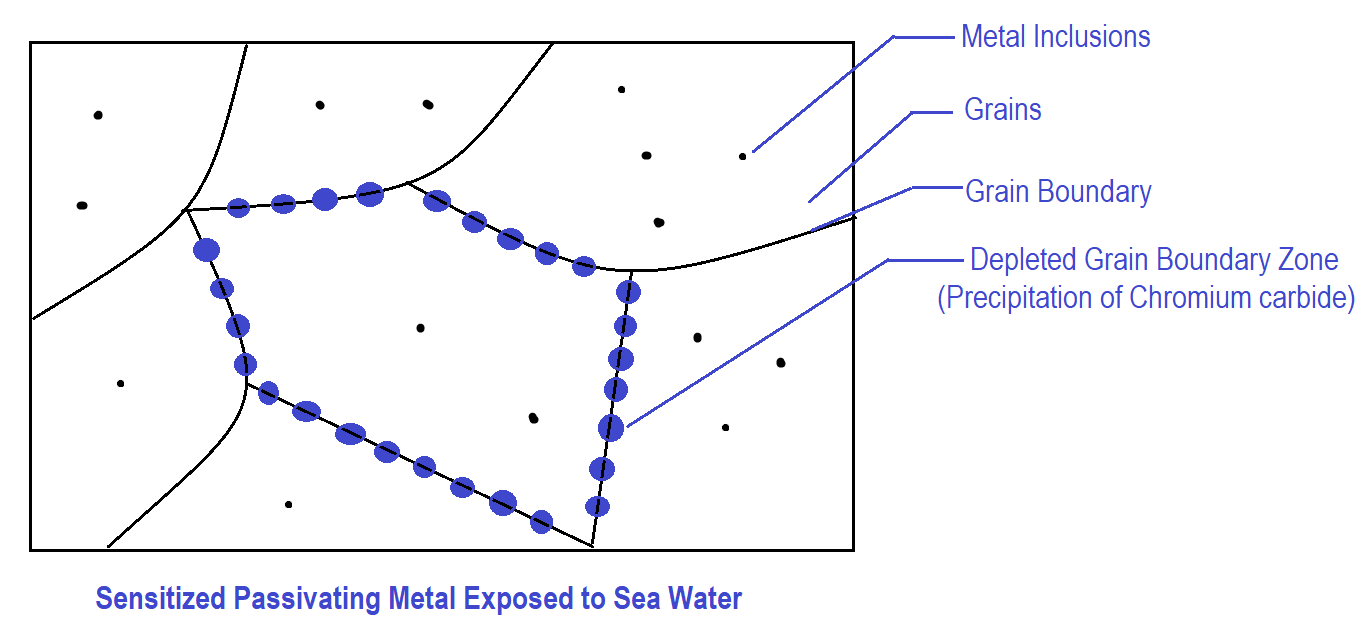
- The metallic surface is in a sensitized condition. (Chromium depletion in grain boundary zone)
Pitting Corrosion Mechanism
Pitting corrosion of stainless steel occurs in three stages. They are
- Pit initiation
- Pit propagation (Autocatalytic reaction takes place)
- Pit termination (Resulting in leakage)
Pit Initiation in Pitting Corrosion of Stainless Steel
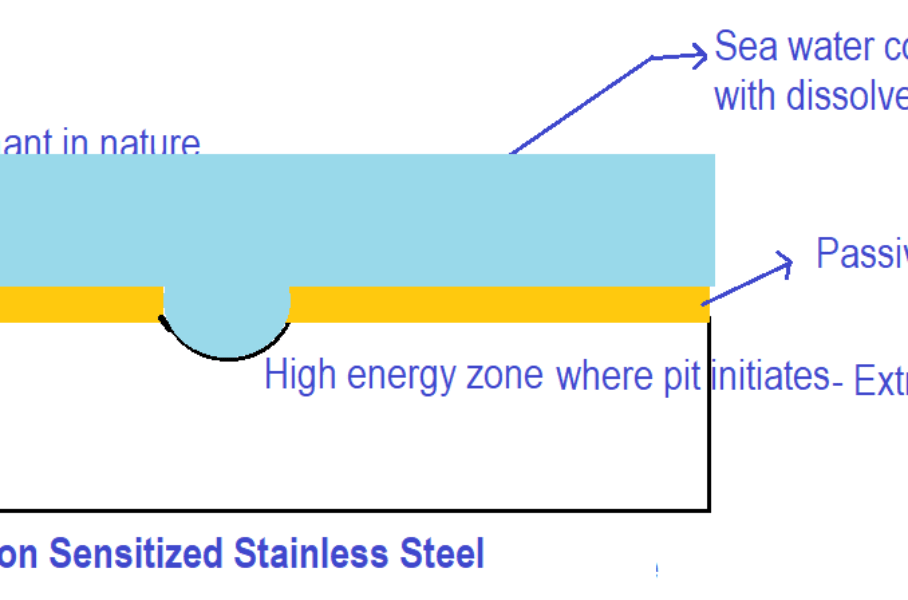
Consider a sensitized stainless-steel surface exposed to seawater having sodium chloride and dissolved oxygen as shown in the figure below.
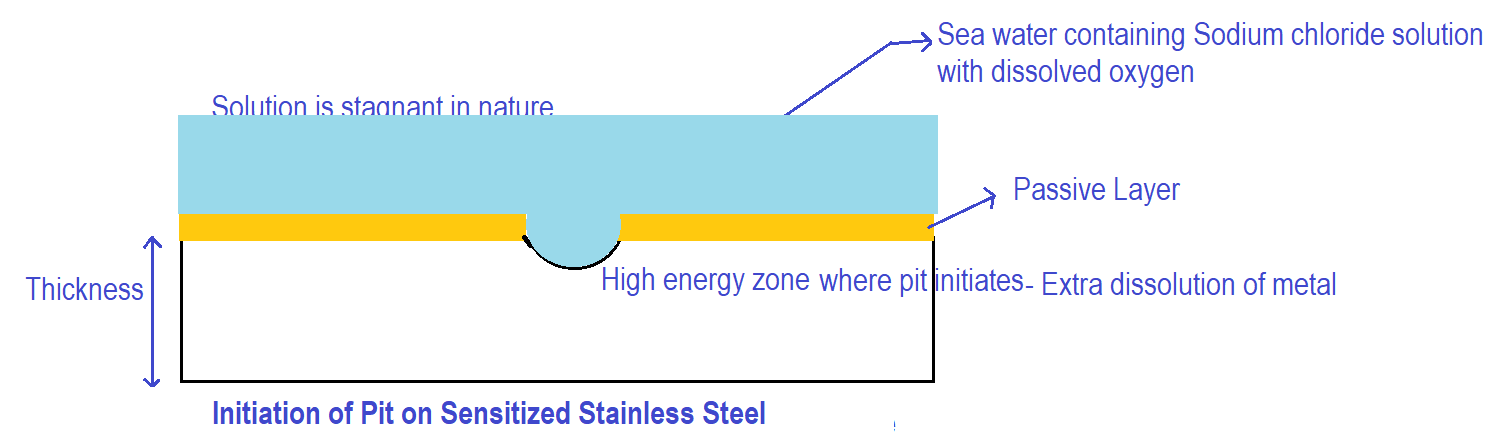
Initially, when the stainless-steel surface is exposed to the seawater as shown in the figure above, dissolution (i.e. oxidation of metal) takes place throughout the surface. The stainless-steel surface considered above has high-energy regions like inclusions & some depleted zones (due to sensitized conditions) and grain boundary regions. Due to the presence of high-energy regions, there could be a momentarily huge rate of corrosion at high-energy zones leading to the initiation of pits on the metal surface.
Pitting Corrosion Reaction
Anode Reaction (Metal Dissolution):
- The location inside the Pit acts as an anode. At the anode, the exposed stainless steel undergoes oxidation. This means it loses electrons and dissolves into the surrounding electrolyte (i.e. sea water).
- Reaction: Fe (iron from stainless steel) →
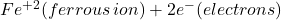
Cathode Reaction (Oxygen Reduction):
- The location on the surrounding stainless-steel surface outside the pit acts as a large cathode.
- The electrons released from the dissolving metal (anode) need to flow somewhere to complete the electrical circuit. In an oxygenated environment (dissolved oxygen in the seawater), a common cathode reaction involves oxygen reduction.
- Reaction:
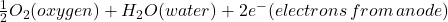 →
→ 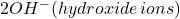 .
.
When the pit is initiated, there is the presence of more and more stagnant solution above the pit. But the liquid above the surface adjacent to the pit is less stagnant. Near the pit due to continuous oxidation, oxygen depletes. Due to stagnation, oxygen from the bulk liquid cannot go to the pit zone. Hence there is the formation of oxygen-rich and oxygen-depleted zones on the metal thus forming the concentration cells as shown in the figure below.
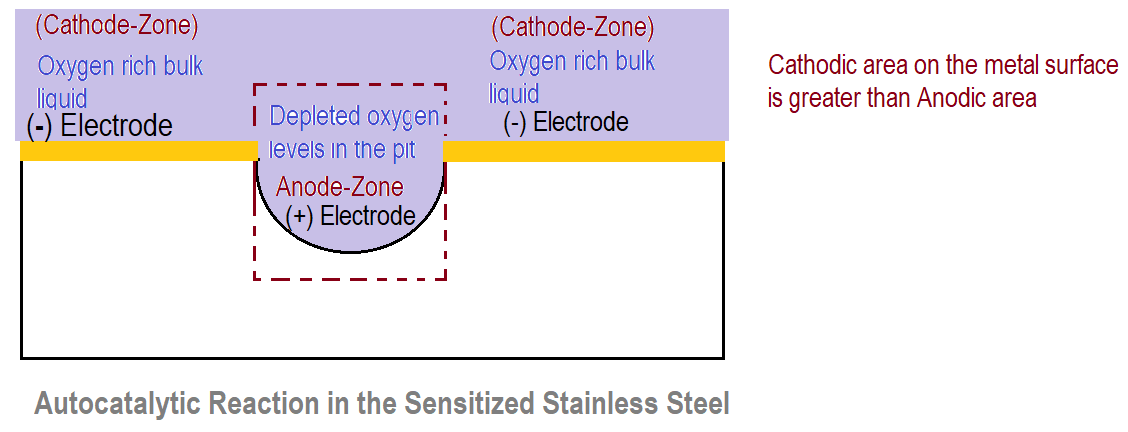
Oxygen oxygen-rich zone acts as a cathode and oxygen oxygen-depleted zone i.e. near the pit acts as an anode. As compared to the overall surface area, the cathode area is very large as compared to the anode area. Hence more anodic dissolution occurs in the pit due to the large potential difference between anode and cathode.
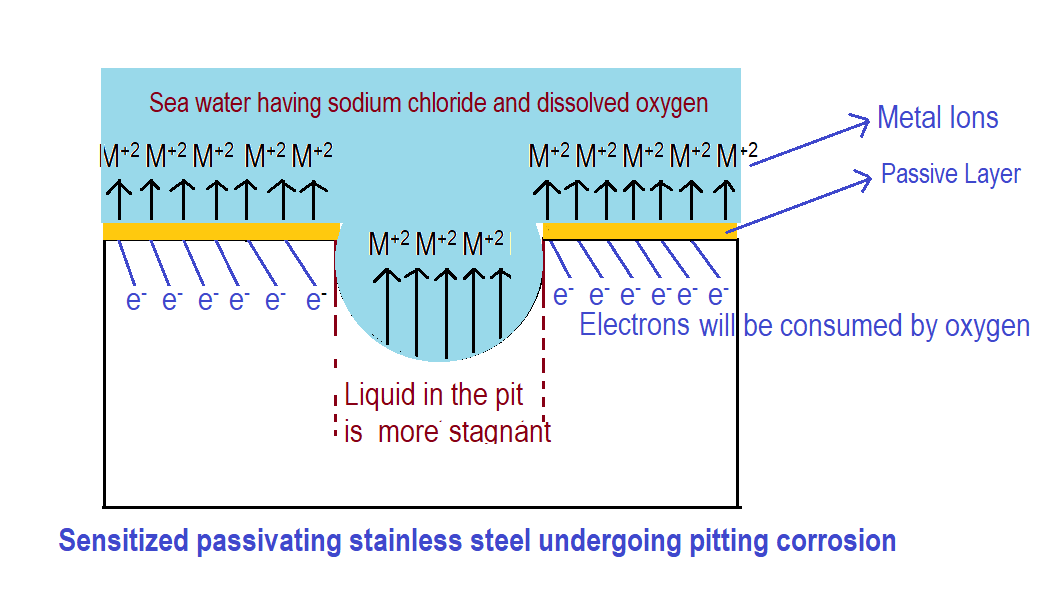
![]() ions from the seawater come to the pit to neutralize the extra positive charges of metal ions
ions from the seawater come to the pit to neutralize the extra positive charges of metal ions ![]() . Metal ions react with chloride ions as follows.
. Metal ions react with chloride ions as follows.
![]()
Thus, Hydrogen ion concentration in the pit increases, the pH value reduces and the solution in the pit becomes highly acidic resulting in the breakdown of the passive layer. It becomes an autocatalytic reaction.
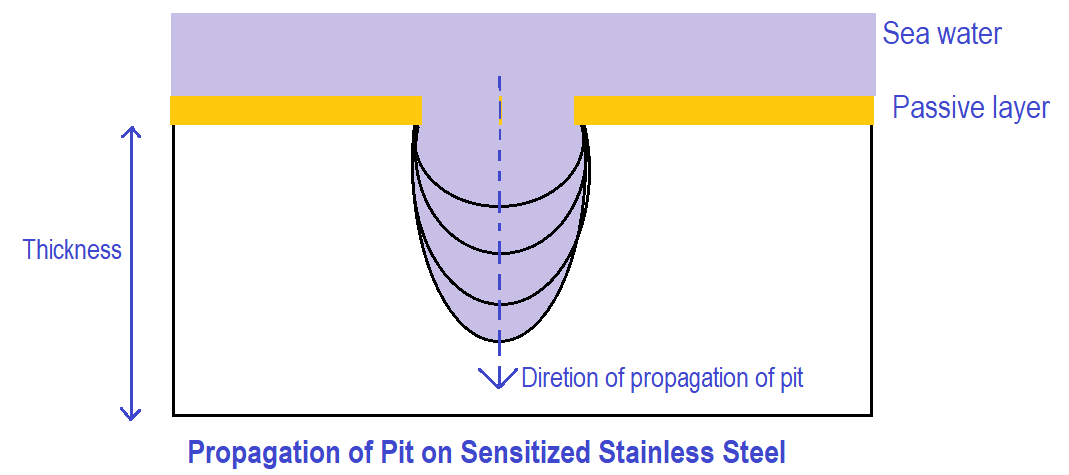
Propagation of Pit Pitting Corrosion of Stainless Steel
Due to autocatalytic reaction, the pit corrosion aggravates and the pit increases in the direction of the thickness of metal as shown in the figure below.
Pit Termination Pitting Corrosion of Stainless Steel
Dissolution goes in the direction of depth at a faster rate and gradually metal gets perforated. Finally, the pit crosses the complete thickness of metal and leads to leakage of the working fluid.
Pitting Corrosion Prevention
Pitting corrosion in stainless steel can be reduced by
- Make the passive layer more stable, more adherent to the parent metal surface and stronger by adding alloying elements like Molybdenum in stainless steel.
- Preventing the stagnancy of working fluid in contact with the stainless steel. If there is no stagnancy, there is no oxygen depletion near the pit, hence no concentration cell formation and no anodic dissolution.
It is advised that fluid movement on the metal surfaces should always be ensured for the SS impellers in centrifugal pumps, and cooling water tubes in heat exchangers to avoid puncturing.
Pitting Corrosion Test for Stainless Steel
ASTM G48 provides standard test methods for evaluating resistance to initiation of pitting corrosion of stainless steels by using a Ferric Chloride solution.
- Sample Preparation: A test specimen is prepared from the material being evaluated.
- Ferric Chloride Bath: The specimen is submerged in a solution of ferric chloride at a specific temperature (usually 22°C or 50°C for stainless steel).
- Exposure Time: The specimen is typically exposed for 24 to 72 hours.
- Evaluation: After exposure, the specimen is cleaned and examined for signs of pitting corrosion. This can involve visual inspection, weight loss measurements, and microscopy.
Allowable Chlorine and Chloride Content in Stainless Steel
Allowable chlorine and chloride content in fluid contacting SS304L and SS316L to avoid pitting corrosion is given below.
| Material | Allowable Chlorine Content ( | Allowable Chloride Content ( |
| SS304L | Less than 2 PPM | Less than 100 PPM |
| SS316L | Less than 4 PPM | Less than 2000 PPM |
FAQ
Why grain boundary regions are called high-energy regions?
Within the grains, atoms bond with neighbouring atoms in an ordered manner since metal is a crystalline structure. But at the grain boundary, there is a mismatch in the orientation of grains resulting in less efficient packing of atoms between two grains at grain boundary. Thus, grain boundary zones are less stable energetically as compared to grains, hence they are called high-energy regions. Any metallic inclusions in the metal can also disrupt the ordered arrangement of atoms, thus they also act as high-energy zones.
What is sensitized stainless steel?
Sensitization happens when chromium carbides precipitate at the grain boundaries of the stainless steel, reducing the chromium content in those regions. A minimum of 10.5 % Chromium is crucial for stainless steel to develop a Chromium Oxide protective layer on its surface for corrosion resistance, so its reduction at the grain boundaries makes the stainless steel sensitive to intergranular corrosion.