Table of Contents
What is Solution Annealing?
The term ‘solution’ represents “solid solution” and annealing is a type of heat treatment that represents heating metal up to a certain temperature and giving sufficient time for the atoms to arrange properly in an orderly fashion such that they are stable. A solid solution is a phase where two or more elements are completely soluble in each other.
Solution Annealing Process
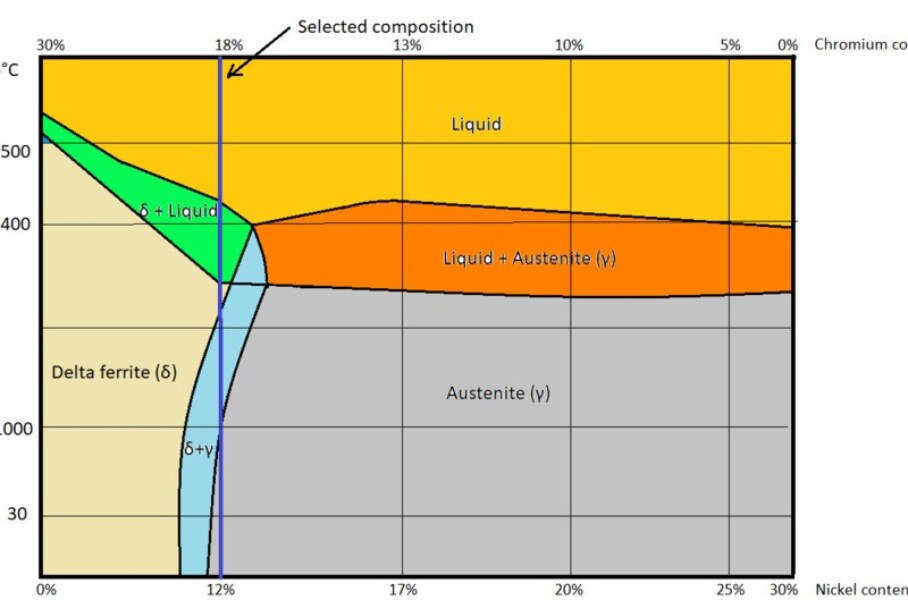
Let us take an example to understand the solution annealing. Consider SS304L metal of composition 70% iron by weight and the remaining 30 % is the combination of chromium and nickel. Its composition is shown in the blue line in the following SS304L phase diagram where chromium is 18 % and nickel is 12%.
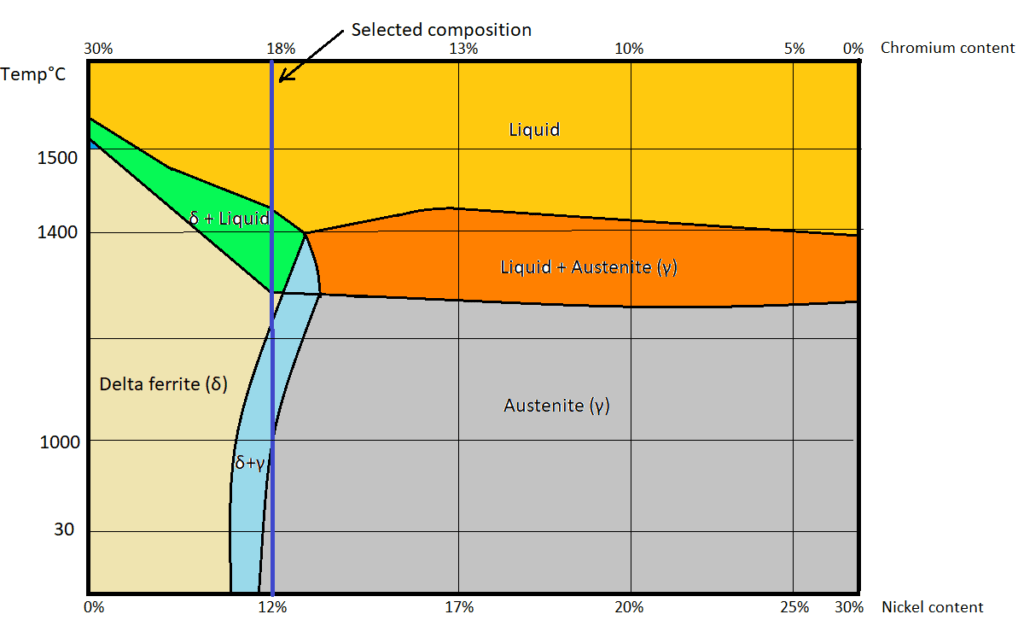
Assume the metal is in the molten state and allow it to cool to room temperature. When the metal starts cooling or solidifying, it follows the blue line path as shown in the above figure.
Examining the phase diagram of SS304L
At 1500 ![]() the chosen metal is in liquid state.
the chosen metal is in liquid state.
Once it starts cooling, molten metal reaches the delta ferrite + liquid zone. And upon cooling, it reaches the delta ferrite + austenite zone.
During the welding of a thin section of SS 304L, the transition from 1500 ![]() to 1050
to 1050 ![]() (as shown in the above figure) happens in a fraction of seconds. (Because thin sections will have less volume as compared to the thick sections of the same area. The higher the volume of metal, the higher the molten metal deposition during welding, the higher the stored heat content, slower the cooling time. During such a fast transition, delta ferrite formed in the two-phase region cannot be transformed into the austenitic phase due to a lack of sufficient diffusion time for the atoms. As per the above-shown figure when the metal cooled to room temperature i.e., 30
(as shown in the above figure) happens in a fraction of seconds. (Because thin sections will have less volume as compared to the thick sections of the same area. The higher the volume of metal, the higher the molten metal deposition during welding, the higher the stored heat content, slower the cooling time. During such a fast transition, delta ferrite formed in the two-phase region cannot be transformed into the austenitic phase due to a lack of sufficient diffusion time for the atoms. As per the above-shown figure when the metal cooled to room temperature i.e., 30 ![]() , the metal should be in the austenitic phase. But in reality, after welding, the metal is in the two-phase zone (i.e., the mixture of delta ferrite and austenite phase) because the above-shown phase diagram is an equilibrium diagram. So due to the sudden transition from 1500
, the metal should be in the austenitic phase. But in reality, after welding, the metal is in the two-phase zone (i.e., the mixture of delta ferrite and austenite phase) because the above-shown phase diagram is an equilibrium diagram. So due to the sudden transition from 1500 ![]() to 1050
to 1050 ![]() , it cannot be called as equilibrium cooling thus it cannot be represented in equilibrium diagram.
, it cannot be called as equilibrium cooling thus it cannot be represented in equilibrium diagram.
Benefits of Solution Annealing
To avoid resulting in a two-phase structure, solution annealing heat treatment is used to convert it into a single-phase solid solution.
Importance of Temperature in Solution Annealing
In solution annealing, metal is heated to 1050 ![]() because 1050
because 1050 ![]() is the optimum temperature to retain the mechanical stability of metal and to allow the movement of atoms to transform from ferrite phase to austenite phase.
is the optimum temperature to retain the mechanical stability of metal and to allow the movement of atoms to transform from ferrite phase to austenite phase.
Rapid Quenching in Solution Annealing
After residing at 1050 ![]() (up to the time of complete transformation of left-over ferrite to austenitic structure) metal undergoes rapid quenching to arrest the diffusion of atoms to go back to the delta phase. Thus, instead of two different phases, it resulted in a single-phase austenitic structure i.e., a solid solution. For the chosen composition of the metal, at room temperature, the austenitic phase is the most stable phase. In general, water is used as a quenching medium.
(up to the time of complete transformation of left-over ferrite to austenitic structure) metal undergoes rapid quenching to arrest the diffusion of atoms to go back to the delta phase. Thus, instead of two different phases, it resulted in a single-phase austenitic structure i.e., a solid solution. For the chosen composition of the metal, at room temperature, the austenitic phase is the most stable phase. In general, water is used as a quenching medium.
Due to solution annealing heat treatment, other solute atoms like titanium…etc will be dissolved in a solid solution resulting in a single-phase structure instead of getting precipitated.
Difference between Annealing and Solution Annealing
Annealing
Annealing is a heat treatment process where a metal is heated to a certain temperature ( for steels, the annealing temperature ranges from 750 to 900 ![]() ) and then cooled slowly. The metal stays inside the furnace. The furnace is turned off, and both the metal and the furnace cool down naturally with the help of the air. It takes a longer time to cool the metal thus having sufficient time for self-aligning the atoms in an orderly manner. This makes the material softer and more flexible. It also helps to reduce residual stress built up inside the material, making it easier to work with and refines its structure.
) and then cooled slowly. The metal stays inside the furnace. The furnace is turned off, and both the metal and the furnace cool down naturally with the help of the air. It takes a longer time to cool the metal thus having sufficient time for self-aligning the atoms in an orderly manner. This makes the material softer and more flexible. It also helps to reduce residual stress built up inside the material, making it easier to work with and refines its structure.
Solution Annealing
On the other hand, solution annealing is a specific type of annealing used mainly for certain metal alloys like stainless steel and nickel alloys. It’s done to dissolve and evenly spread alloying elements within the base metal. This enhances the metal’s ability to resist corrosion, improves its strength, and makes it more stable.