Table of Contents
Interview Questions Part-4 (16 to 20)
16. What makes welding of aluminium difficult?
We have to know the characteristics of the aluminium to judge the difficulty level in welding aluminium metal. Welding of aluminium metal is, in a sense joining of two aluminium metal pieces permanently.
Following are the two main characteristics of aluminium that make it difficult to weld.
Characteristic-1: Oxidation
At high temperatures, aluminium metal has a high affinity towards oxygen, resulting in forming an aluminium oxide layer on the top surface while carrying out welding activity. The melting point of aluminium oxide layer is ![]() 2000
2000 ![]() but the melting point of pure aluminium metal is 600
but the melting point of pure aluminium metal is 600 ![]() . This aluminium oxide layer on the top surface obstructs the heat transfer from the welding torch, resulting in insufficient heat transfer to melt the base metal located beneath the oxide layer, making aluminium difficult to weld.
. This aluminium oxide layer on the top surface obstructs the heat transfer from the welding torch, resulting in insufficient heat transfer to melt the base metal located beneath the oxide layer, making aluminium difficult to weld.
Characteristic-2: Porosity
At high temperatures, aluminium absorbs Hydrogen from the atmosphere. When the metal cools down, it leaves empty spaces called pores in the metal. The formation of these pores is called porosity.
17. Which type of welding is preferred for welding aluminium metal?
Based on the two significant characteristics of aluminium metal as explained in question no.16, Gas Tungsten Arc Welding (GTAW), also known as Tungsten Inert Gas (TIG) welding is the preferred type because of the following reasons.
Corrective action for oxidation
Tungsten Inert Gas welding using Alternating Current (AC) is preferred for welding aluminium metals. TIG with Direct Current DC is preferred for welding mild/ stainless steel. Each cycle of AC consists of two parts, i.e., positive half and a negative half, as shown in the following figure.
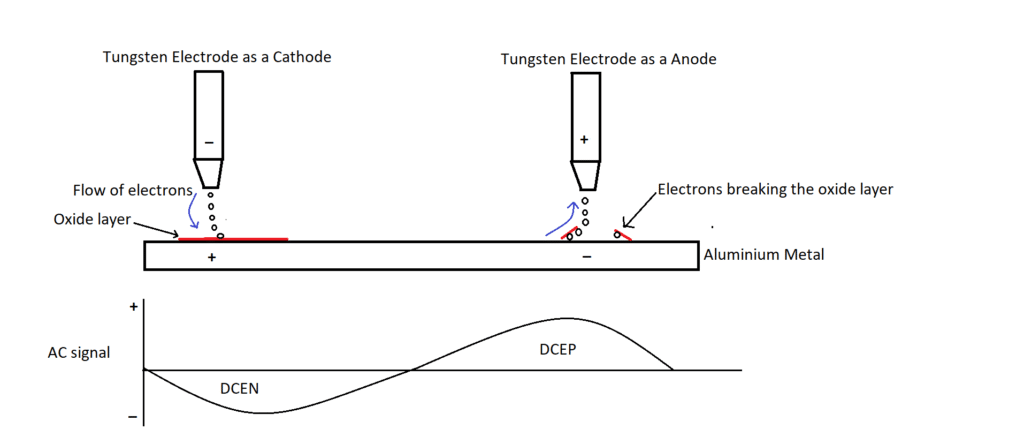
In the negative half cycle, the tungsten electrode will be negative (acts as a cathode), and the base metal will be positive (acts as an anode). Therefore, it can be treated as Direct Current Electrode Negative (DCEN). (Electrons always flow from the negative terminal to the positive terminal). In DCEN, electrons flow from the electrode to the base metal resulting in 2/3rd of heat generation in the base metal and 1/3rd of heat generation in the electrode. Thus, in the negative half cycle, the temperature of the base metal increases and an aluminium oxide layer will form.
Similarly, the next half-cycle, i.e., positive half-cycle, is similar to DCEP, but electrons flow from the base metal to the electrode since the charge is changed in the next half of the AC cycle. While the electrons move out from the base metal, electrons break the oxide layer on the top surface, as shown in the above figure.
Thus, it melts the base metal in the half-cycle, and in the next half-cycle, it cleans the oxide layer. This phenomenon is called the self-cleaning characteristic. This characteristic helps in transferring the heat effectively from the arc to the base metal.
Corrective action for porosity
Shielding: Inert gas, i.e., Argon, is used as a shielding gas to avoid the contact of molten weld metal to the atmosphere.
18. What is the difference between welding and soldering?
| Welding | Soldering |
| Base metal melts to get fused along with the filler metal | The base metal will not melt. Only the filler metal melts |
| Forms the strongest joint | A relatively weaker joint as compared to welding |
| Welding temperatures is greater than 400 | Soldering temperature is less than or equal to 400 |
| Filler metal composition is more or less equivalent to the base metal. | Lead + tin is called solder. Melting of solder varies between 90 to 400 |
| It is used to join the mechanical components. | It is used to join the electronic components to a circuit board. |
19. Why is mild steel called “mild” steel?
Generally, a misconception is, mild steel is called “mild” because of its low carbon content. Mild means “less” or “no”. Mild steel represents a mild response to the heat treatment, and especially it is impossible to get a Martensitic crystal structure for mild steel. It is explained with the help of the Time-Temperature-Transformation (TTT) curve of mild steel.
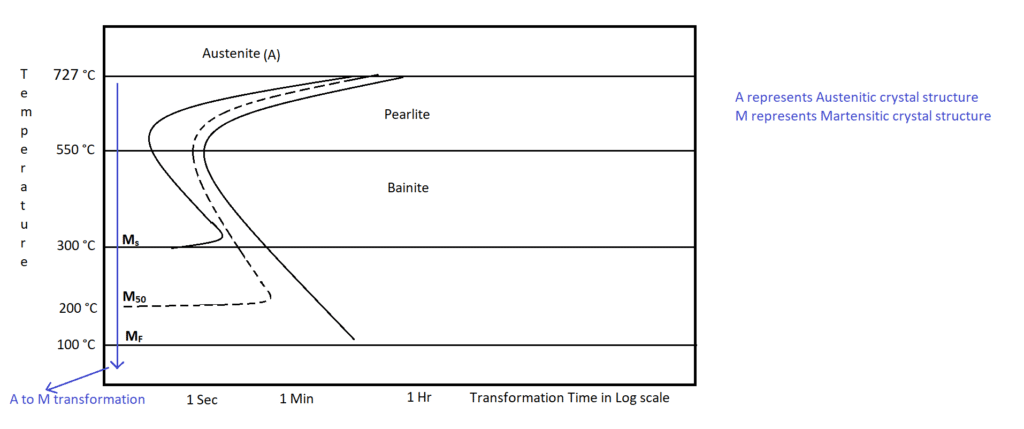
The blue line from the above figure represents the formation of a martensitic structure from an austenitic structure. If we cool (quench in water) the mild steel from austenitic temperature (i.e.,727 ![]() ) to complete Martensitic transformation temperature in less than one second, i.e., 1000
) to complete Martensitic transformation temperature in less than one second, i.e., 1000 ![]() per second cooling rate then the resultant crystal structure of mild will be martensite. But practically, such an ultra-high cooling rate is impossible to achieve. Therefore, mild steel display a mild response to heat treatment.
per second cooling rate then the resultant crystal structure of mild will be martensite. But practically, such an ultra-high cooling rate is impossible to achieve. Therefore, mild steel display a mild response to heat treatment.
20. What is the difference between boiling and evaporation?
| Boiling | Evaporation |
| Transformation of liquid to vapour state | Conversion of liquid to vapour state |
| Occurs at the solid-liquid interface (i.e., Between the bottom-most part of the hot beaker and the bottom-most layer of water) | Occurs at the liquid-vapour interface. (i.e., Between the topmost layer of water and the atmosphere) |
| Involves bubble formation | No bubble formation |
| External heating is required | Heating is not required |
| Relatively faster process since external heating is involved | Somewhat slower since the transformation is solely due to the difference in vapour pressure of atmosphere and saturation pressure of water at a given temperature. |
| The temperature of liquid remains constant because once the boiling point is reached, the heat supplied is utilized to convert the liquid into vapour instead of increasing the temperature of the liquid. | The temperature of the liquid body decreases. Just like the feel when the sweat evaporates from the body. |
Boiling
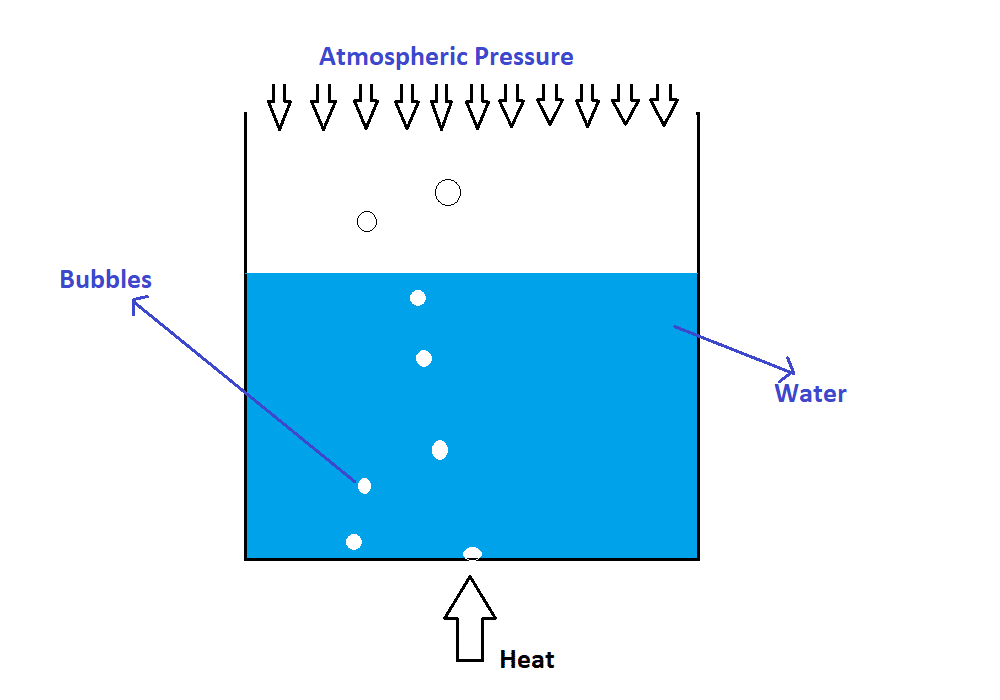
Consider heating the water-filled in the open-top beaker opened to the atmosphere as shown in the following figure (i.e., heating the water at 1 atm pressure). The process of bringing the water to a temperature such that bubble formation starts and turns into vapour is called boiling. When the water temperature reaches 100 ![]() the bottom-most layer receives the highest amount of heat. Hence, water at the bottom-most layer turns into vapour, i.e., a bubble (that’s why the bubble starts from the bottom of the beaker) begins to form at the solid-liquid interface. The bubble starts to rise when the vapour pressure (i.e., vapour within the liquid is called a bubble. Of course, the vapour will have the pressure) is greater than or equal to the atmospheric pressure, as shown in the following figure.
the bottom-most layer receives the highest amount of heat. Hence, water at the bottom-most layer turns into vapour, i.e., a bubble (that’s why the bubble starts from the bottom of the beaker) begins to form at the solid-liquid interface. The bubble starts to rise when the vapour pressure (i.e., vapour within the liquid is called a bubble. Of course, the vapour will have the pressure) is greater than or equal to the atmospheric pressure, as shown in the following figure.
Evaporation
Consider a beaker filled with water, and the top is open to the atmosphere but with no external heating.
The process of transforming liquid into vapour when the vapour pressure of atmospheric air is less than the saturation pressure of water at a given temperature is called evaporation.
Note: Atmospheric air is a mixture of dry air and water vapour i.e., ![]() . Vapour in the atmosphere is due to the presence of water in the vapour form. When the vapour pressure of the atmospheric air is less, it indicates that air contains less moisture. Due to the pressure difference, the molecules in the topmost layer of water move to the atmospheric air, which is known as evaporation. Thus, evaporation depends on the humidity (i.e., moisture) as well as the vapour pressure of the atmospheric air. Once the air is saturated, it cannot hold any additional water vapour because it contains sufficient water. Thus, water cannot move to the atmosphere from the liquid surface; hence there is no pressure difference as well. It indicates that the vapour pressure of saturated air is equal to the saturation pressure of water at that temperature.
. Vapour in the atmosphere is due to the presence of water in the vapour form. When the vapour pressure of the atmospheric air is less, it indicates that air contains less moisture. Due to the pressure difference, the molecules in the topmost layer of water move to the atmospheric air, which is known as evaporation. Thus, evaporation depends on the humidity (i.e., moisture) as well as the vapour pressure of the atmospheric air. Once the air is saturated, it cannot hold any additional water vapour because it contains sufficient water. Thus, water cannot move to the atmosphere from the liquid surface; hence there is no pressure difference as well. It indicates that the vapour pressure of saturated air is equal to the saturation pressure of water at that temperature.
![]()
Where,
![]() represents the relative humidity
represents the relative humidity

